The Relationship Between Pressure and Temperature (Gay-Lussac’s Law)
The Gay-Lussac's law is a simple gas law.
Gay-Lussac’s Law
Be careful!
Simple gas laws apply only for ideal gases.
In other words, the values calculated using simple gas laws give us an approximation of the values that would be measured in reality. As long as the temperature is not too low and the pressure is not too high, these approximate values are very useful for predicting all kinds of situations.
Gay-Lussac's law describes the relationship between the pressure |(P)| and the temperature |(T)| of a gas.
The following images show a certain amount of gas in a container of constant volume.
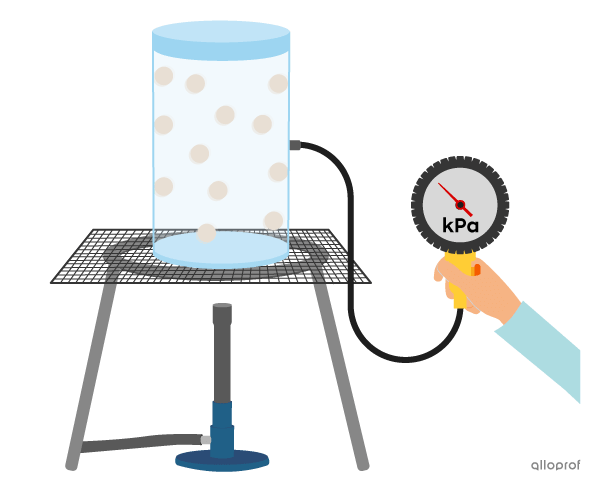
At a given temperature, the particles in the gas exert a certain pressure. This pressure is caused by the particles colliding with the walls of the container.

If the temperature of the gas is increased by heating, the kinetic energy of the particles also increases. The particles go into more collisions, so the pressure on the walls of the container increases.
Move the slider up and down to control the gas temperature, then observe the effect on the pressure.
In short, if the number of moles |(n)| and the volume |(V)| are constant, increasing the temperature |(T)| of a gas increases the pressure |(P)| it exerts. On the other hand, when the temperature decreases, the pressure decreases.
Pressure is said to be directly proportional to temperature. This relationship can be expressed using the following formula.
When |V| and |n| are constant:
|P\propto T| or |\dfrac{P}{T}=\text{constant}|
where
|P\ :| pressure often in kilopascals |(\text{kPa})|
|T\ :| temperature in kelvins |(\text{K})|
We can also compare an initial situation and a final situation in which the temperature and pressure of a gas change. The following formula can then be used.
When |V| and |n| are constant:
|\dfrac{P_1}{T_1}=\dfrac{P_2}{T_2}|
where
|P_1\ :| initial pressure often in kilopascals |(\text{kPa})|
|T_1\ :| initial temperature in kelvins |(\text{K})|
|P_2\ :| final pressure often in kilopascals |(\text{kPa})|
|T_2\ :| final temperature kelvins |(\text{K})|
When driving a car, the friction between the road and the tires tends to heat them and the air they contain. This affects the air pressure in the tires.
Before driving the car, we assume that the air temperature in the tire is the same as the outside temperature, i.e. |22{,}00\ ^\circ\text{C}.| Using a manometer, we measure the initial air pressure in one of the tires and obtain |220{,}63\ \text{kPa}.|
After driving for a while, the manometer reads |234{,}42\ \text{kPa}.|
We assume that the amount of air |(n)| in the tire stays the same and we neglect the change in volume |(V),| determine the variation of the air temperature in the tire.

Gay-Lussac’s Law - Example
See solution
Video
Video coming soon!
Exercice
Exercise coming soon!
See Also
- The Kinetic Theory of Gases
- Simple Gas Laws
- The Relationship Between Volume and Temperature (Charles’s Law)
- The Relationship Between Volume and Pressure (Boyle’s Law)
- The Relationship Between Volume and Number of Moles (Avogadro’s Law)
- The Relationship Between Pressure and Number of Moles
- The General Gas Law
- The Ideal Gas Law
- The Law of Partial Pressures (Dalton’s Law)
