Density | Elementary
Density is a property of matter. It influences the buoyancy of objects.
What is density?
The density of an object is the ratio of its mass to its volume. It is measured in grams per millilitre (g/mL).
-
The mass of an object is the amount of matter it contains.
-
The volume of an object is the space it occupies.
-
Density is the amount of matter contained in a given space.
All substances have a density. The following image shows you some examples of substances and their densities.

The density of various substances
According to the image, the density of liquid water is 1.0 gram per millilitre (1.0 g/mL). This means that a small cube of water with a volume of 1 mL has a mass of 1 g.
The density of a diamond is 3.5 grams per millilitre (3.5 g/mL). This means that a small cube of diamond with a volume of 1 mL has a mass of 3.5 g.

So, in the same volume (the small 1 mL cube), there can be a small amount of matter or a large amount of matter.
When there is a lot of matter in a given volume, the matter is tightly packed together (compact). We say that the substance is dense and that its density is high.
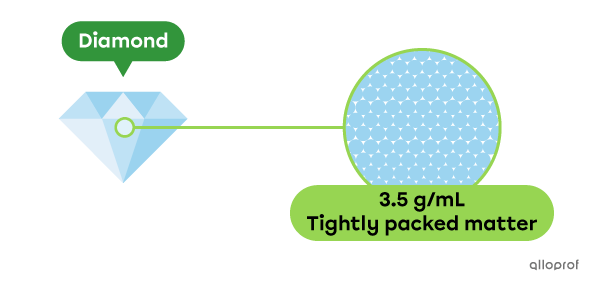
Diamonds are dense. They have a high density.
When there is little matter in a given volume, the matter is spaced out. We say that the substance is not very dense and that its density is low.

Water is less dense than diamond. It has a low density.
Classifying solids of the same volume
Density tells you that even if solids have the same volume, they do not necessarily have the same mass.

These two cubes have the same volume. However, they do not have the same mass. The cube on the left is lighter and also has the lowest density. We say that it is the least dense. The cube on the right is heavier and also has the highest density. We say that it is the most dense.
For example, if you compare this wooden cube and this plastic cube, you will see that:
-
the wooden cube and the plastic cube have the same volume;
-
the wooden cube is lighter (70 g) than the plastic cube (140 g);
-
the density of wood is lower (0.7 g/mL) than the density of plastic (1.4 g/mL).

You place a gold cube on a scale and an aluminum cube on another scale. These two cubes have the same volume.
Which cube has the highest density?

See solution
Classifying solids of the same mass
Density tells you that even if solids have the same mass, they do not necessarily have the same volume. In other words, the same amount of matter can be distributed in a small space or in a large space.

These yellow cubes are solids that have the same mass. However, they do not have the same volume. The largest cube is also the one with the smallest density. We say that it is the least dense. The smallest cube is also the one with the highest density. We say that it is the most dense.
For example, if you compare this diamond and this pile of sand, you will see that:
-
the diamond and the sand have the same mass (35 g);
-
the diamond has a smaller volume than the sand;
-
the density of the diamond is higher (3.5 g/mL) than the density of the sand (1.6 g/mL).

These three objects have the same mass. Classify these objects from lowest to highest density.

