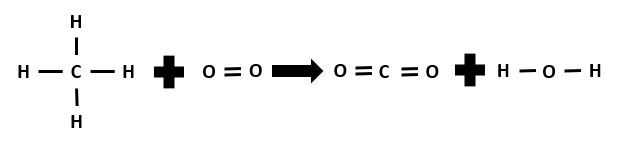The Energy Balance of a Reaction
The energy balance of a reaction is the sum of the energy required to break the chemical bonds of the reactants and the energy released when the bonds of the products are formed.
The aim of a chemical reaction balance is to determine the enthalpy variation of a chemical reaction. There are various ways of doing this.
Energy Balance Based on Chemical Bonds
All chemical reactions involve the breaking of chemical bonds and the formation of new ones. The breaking of chemical bonds always requires an input of energy (positive energy), whereas their formation is accompanied by a release of energy (negative energy). By adding together the energy used to break bonds and the energy required to form them, we can determine whether a reaction is exothermic or endothermic, and thus establish its energy balance.
|\text{Energy balance = Energy absorbed + Energy released}|
Consequently, to determine the overall enthalpy variation of a reaction, we simply add the enthalpy variation associated with the breaking of reactant bonds to that associated with the formation of product bonds. This calculation can be represented by the following formula:
|ΔH = ΔH_{\text{reactants}} + ΔH_{\text{products}}|
Please note: enthalpy variation values are expressed in |\small \text {kJ/mol}|.
In order to draw up an energy balance for a reaction, it is first necessary to take a close look at each of the substances involved in order to identify the types of bonds involved. Each type of bond has its own specific binding energy. The energy of the bond corresponds to the energy required to break it, or to the energy released when it is formed. The link below gives the most common bond energy values.
Find out more!
The following steps can be taken to establish the energy balance of a reaction:
-
determine the type of bonds contained in the substances involved;
-
calculate the energy required to break all the reactant bonds by adding up the energy values for each bond present;
-
calculate the energy associated with the formation of all the chemical bonds in the products;
-
add up these calculated energies to establish the energy balance.
Important!
Note that if the bond is on the reactant side, its sign will be positive. If the bond is on the product side, the sign will be negative.
Calculate the enthalpy change for this reaction by performing an energy balance and determine whether it is an endothermic or exothermic reaction.
|CH_{4(g)} + 2\:O_{2(g)} \rightarrow CO_{2(g)} + 2\: H_{2}O_{(g)}|